SO2, H2O, SO3, and atomic oxygen mixing ratios at 80 km vary with SO2... | Download Scientific Diagram
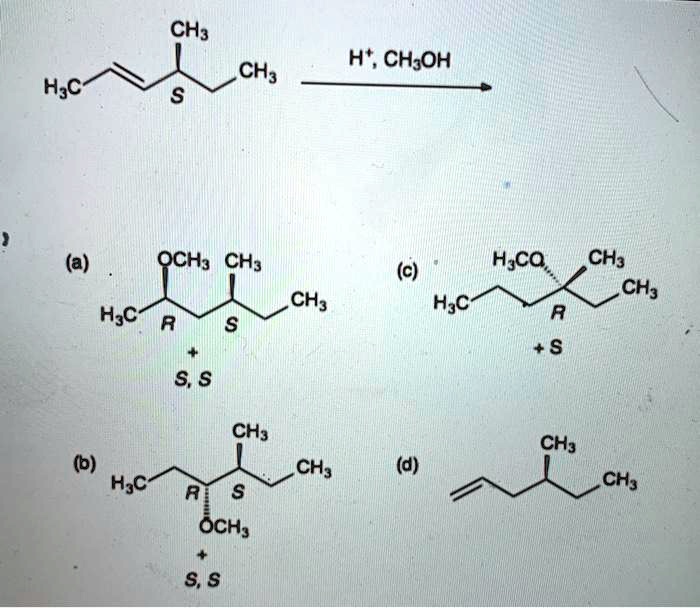
SOLVED: CH3 CH; H2O CH3OH H2O (a) OCH3 CH3 CH3OH S H2O CH3 (c) CH3 H2O a S2O CH3 CH3, al OCH3; CH3 CH3 () H2SO4 S5

Effects of SO2 and H2O on low-temperature NO conversion over F-V2O5-WO3/TiO2 catalysts - ScienceDirect
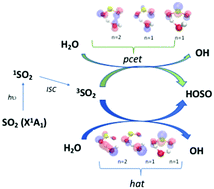
Triplet state promoted reaction of SO2 with H2O by competition between proton coupled electron transfer (pcet) and hydrogen atom transfer (hat) processes - Physical Chemistry Chemical Physics (RSC Publishing)
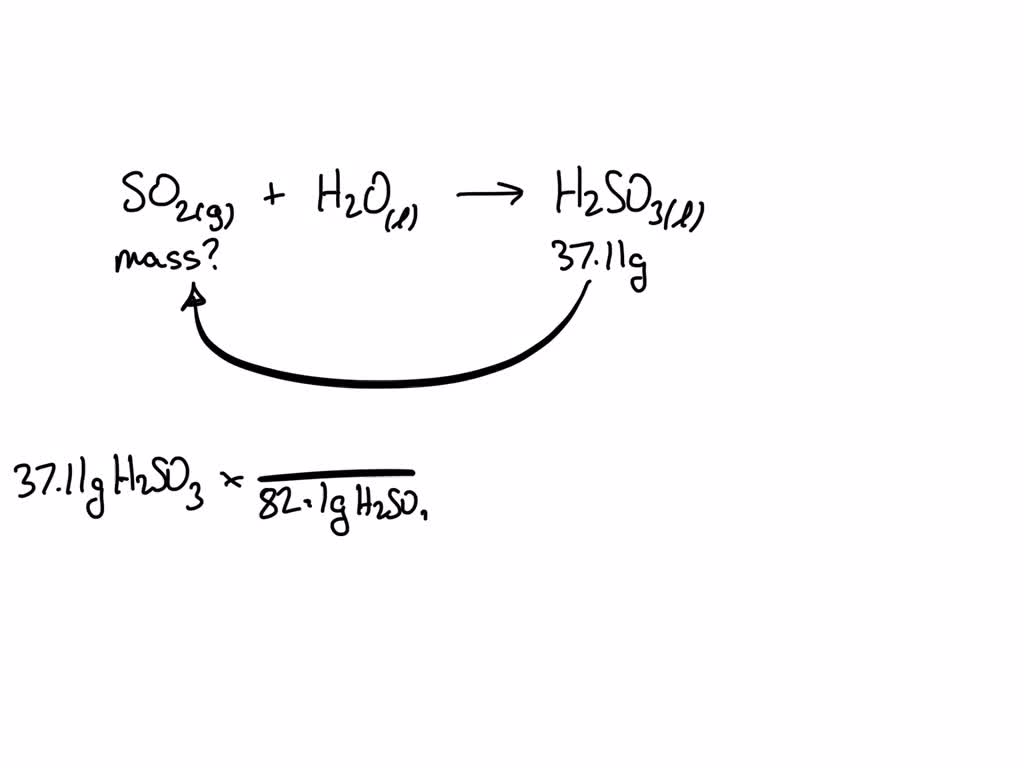
SOLVED: SO2(g) + H2O(l) → H2SO3(l) What mass of sulfur dioxide is needed to prepare 37.11 g of H2SO3?
if S + O2— > SO2 ; ( DH= 298.2 KJ ) SO2 +1/2O2——>SO3 ( DH= 98.7 KJ) SO3 + H2O——>H2SO4 (DH= 130.2 KJ) H2 + 1/2H2O—–> H2O ( DH= 287.3KJ) then

U2 complete the following SO2 + H+ + NO+ +? A SO2 + 2NO+ H2O + N2O+ so YOU MISSED B SO2 + 4NO+2H20 +2N20+5 C SO2 + 2NO+ 4H2O + N20+S4 D None of these

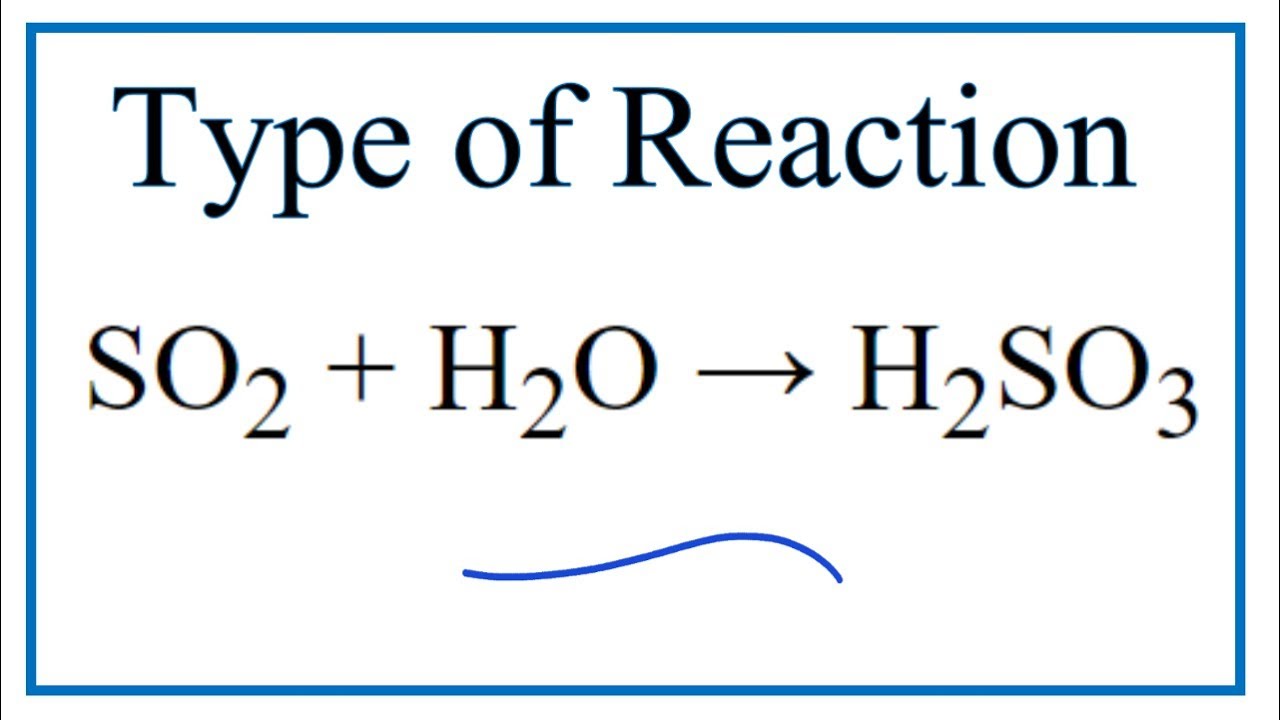
SO2+H2O=H2SO3 balance the chemical equation @mydocumentary838. so2+h2o=h2so3 balance equation. - YouTube