![The number of ions formed on dissolving one molecule of FeSO4(NH4)2SO4 6H2O is/are [1] 6 [2] 3 [3] 5 [4] 4 The number of ions formed on dissolving one molecule of FeSO4(NH4)2SO4 6H2O is/are [1] 6 [2] 3 [3] 5 [4] 4](https://toppr-doubts-media.s3.amazonaws.com/images/6961597/8ca90f38-b3ce-45a2-b804-70850bfc09eb.jpg)
The number of ions formed on dissolving one molecule of FeSO4(NH4)2SO4 6H2O is/are [1] 6 [2] 3 [3] 5 [4] 4

What is ammonium sulfate or (NH4)2SO4? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Ammonium Sulfate [(NH4)2SO4] - Structure, Molecular mass, Preparation, Properties, Uses and FAQs of Ammonium Sulfate. Ammonium Sulfate [(NH4)2SO4] - Structure, Molecular mass, Preparation, Properties, Uses and FAQs of Ammonium Sulfate.](https://cdn1.byjus.com/wp-content/uploads/2018/09/ammonium-sulfate-structure-700x239.png)
Ammonium Sulfate [(NH4)2SO4] - Structure, Molecular mass, Preparation, Properties, Uses and FAQs of Ammonium Sulfate.

SOLVED: The phase diagram for the ternary system NH4Cl / (NH4)2SO4 H2O at 25°C is shown below: 0.2 0.8 P=V 0.4 0.6 P=2 0.6 P=2 0.4 P=3 0.8 0.2 NH4Cl (NH4)2SO4 0.2

Extended (NH4)2SO4/H2O phase diagram. AE and EB are the liquidus and... | Download Scientific Diagram

Effects of seed crystal concentration, pH, and stirring rate on ammonium sulfate crystallization under the action of ammonium nitrate - ScienceDirect
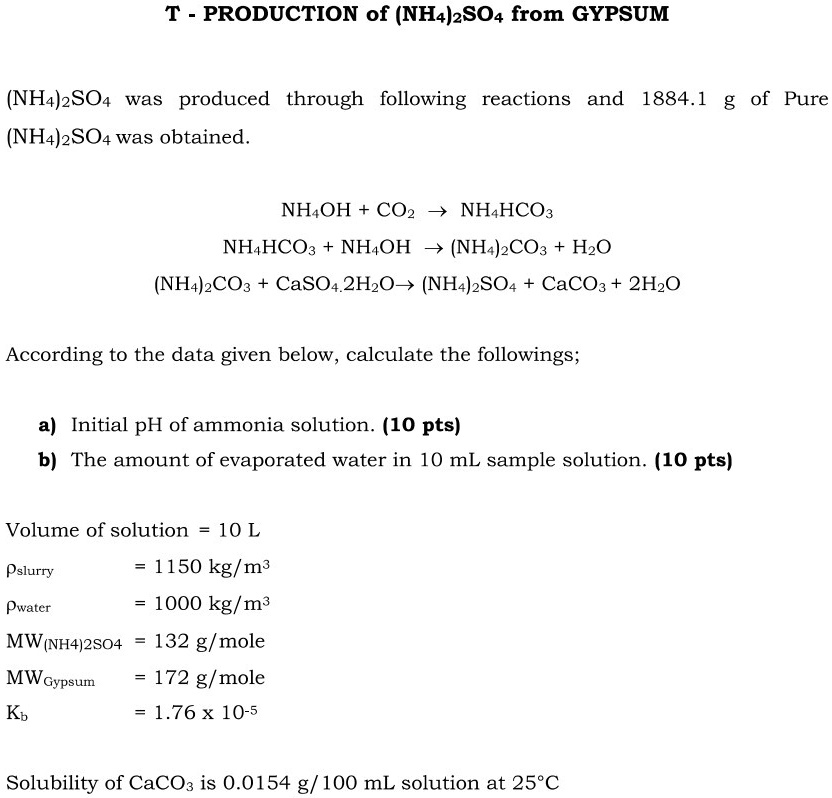
SOLVED: Production of (NH4)2SO4 from GYPSUM (NH4)2SO4 was produced through the following reactions and 1884.1 g of (NH4)2SO4 was obtained. NH4OH + CO2 â†' NH4HCO3 NH4HCO3 â†' NH4OH + CO2 + H2O
![PDF] Phase diagrams of the MgSO4-Al2(SO4)3-(NH4)2SO4-H2O system at 25 and 55 °C and their application in mineral carbonation | Semantic Scholar PDF] Phase diagrams of the MgSO4-Al2(SO4)3-(NH4)2SO4-H2O system at 25 and 55 °C and their application in mineral carbonation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/09b40608810382e919a2a8bad7cda4e5034c2f19/2-Figure1-1.png)
PDF] Phase diagrams of the MgSO4-Al2(SO4)3-(NH4)2SO4-H2O system at 25 and 55 °C and their application in mineral carbonation | Semantic Scholar
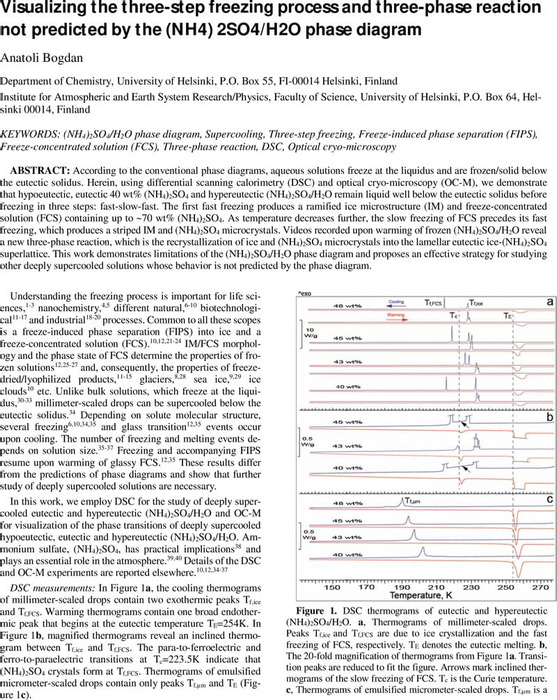
Visualizing the three-step freezing process and three-phase reaction not predicted by the (NH4) 2SO4/H2O phase diagram | Physical Chemistry | ChemRxiv | Cambridge Open Engage

Phase Diagram for the Na2SO4–(NH4)2SO4–MEA–H2O System at Elevated Temperature | Journal of Chemical & Engineering Data

Acid + HNO3 + ....... + Base ....... 2NH,OH salt KNO3 + + Water H2O - - - - (NH4)2SO4 + ........ + KOH KBr +






2SO4.jpg)