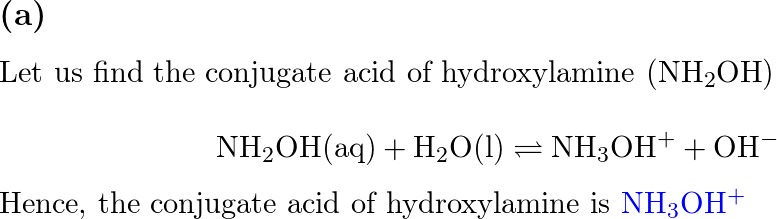Instanton rate constants for reaction ( 4 ) in Model A and Model B .... | Download Scientific Diagram

Is there a pathway for N2O production from hydroxylamine oxidoreductase in ammonia-oxidizing bacteria? | PNAS

Thermal Decomposition of NH2OH and Subsequent Reactions: Ab Initio Transition State Theory and Reflected Shock Tube Experiments | The Journal of Physical Chemistry A

Figure 3 from Formation of hydroxylamine (NH2OH) in electron-irradiated ammonia-water ices. | Semantic Scholar

Scheme 2. Reagents and conditions: (i) NH2OH.HCl, Pyridine, H2O, 24 h,... | Download Scientific Diagram
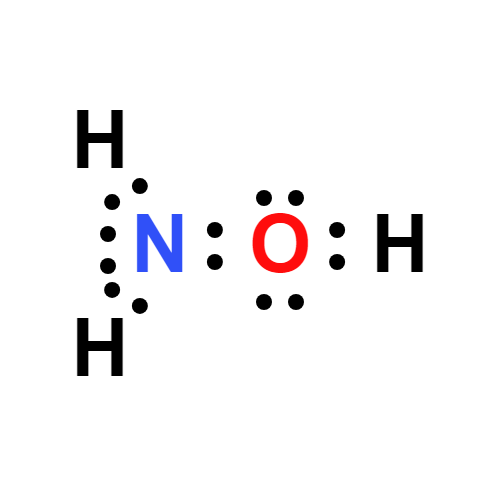
![SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-] SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-]](https://cdn.numerade.com/ask_images/db627e0c62e74e1a9a6bc5925a1a5d0a.jpg)
SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-]
![PDF] Thermal decomposition of NH2OH and subsequent reactions: ab initio transition state theory and reflected shock tube experiments. | Semantic Scholar PDF] Thermal decomposition of NH2OH and subsequent reactions: ab initio transition state theory and reflected shock tube experiments. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b36782da9edaac7d0b91c7a6a1f8caff67f9661e/16-Figure22-1.png)
PDF] Thermal decomposition of NH2OH and subsequent reactions: ab initio transition state theory and reflected shock tube experiments. | Semantic Scholar

Further validation and application of the NH2OH and HNO products of N2 | Download Scientific Diagram
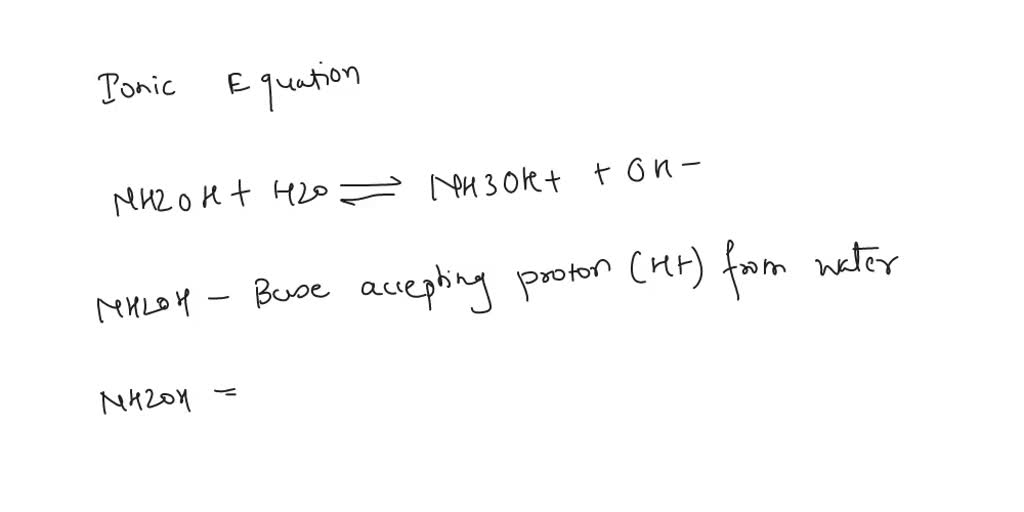
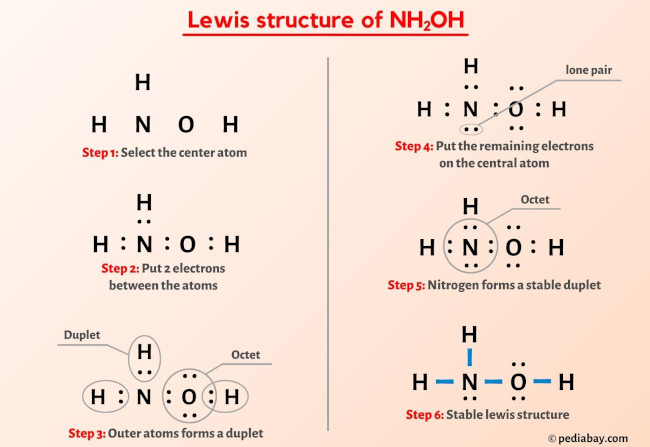


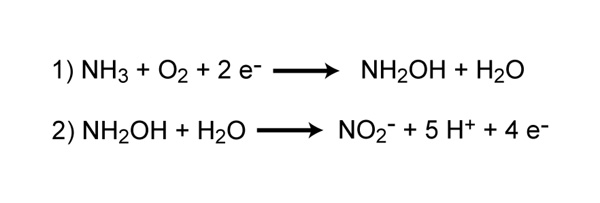
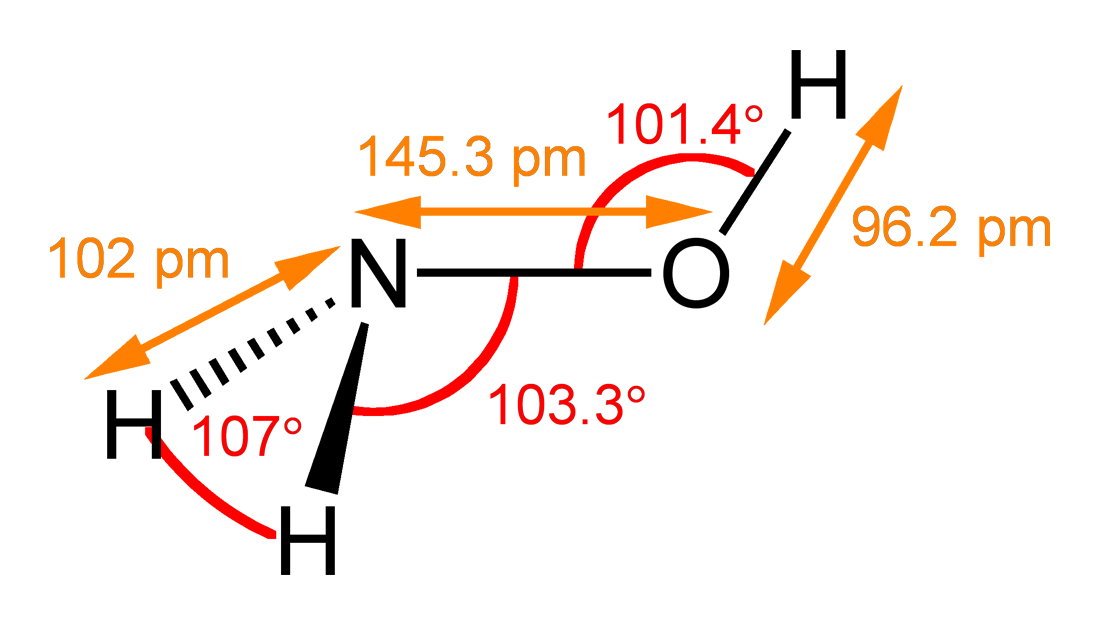

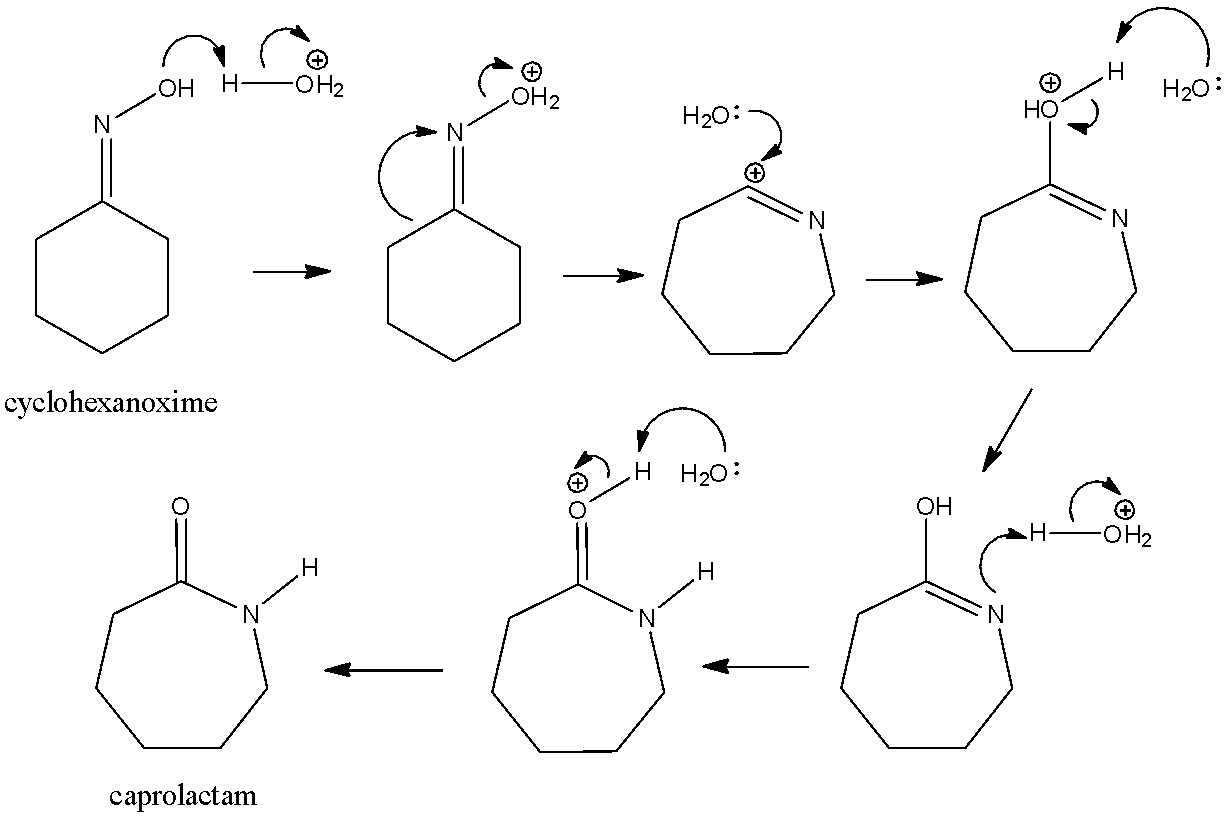
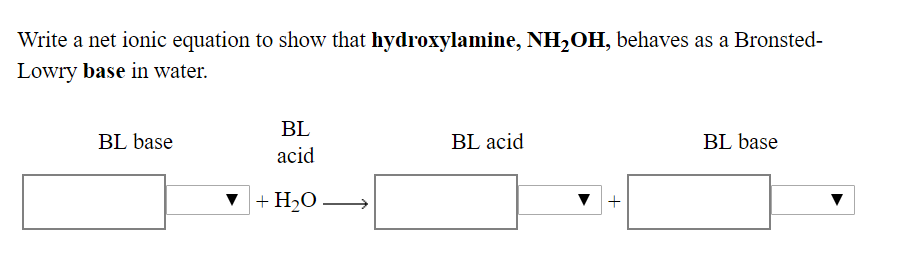

![Malayalam] In the reaction 'A+2B+H2O gives C+2D. draw the structur Malayalam] In the reaction 'A+2B+H2O gives C+2D. draw the structur](https://static.doubtnut.com/ss/web-overlay-thumb/8095007.webp)