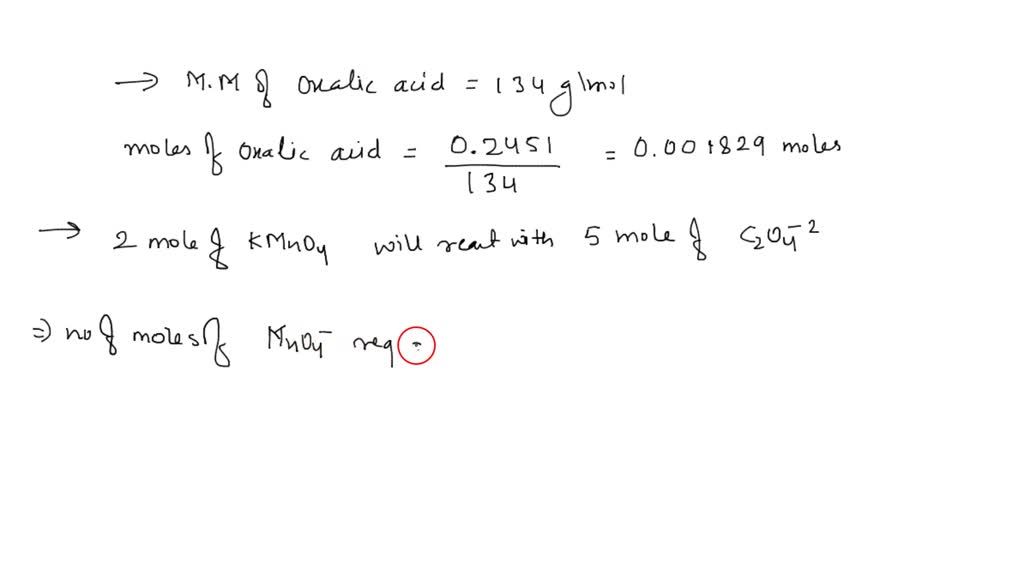
SOLVED: Potassium permanganate solutions may be standardized by titrating against the primary standard sodium oxalate following the reaction: 2 MnO4-(aq) + 5 C2O4^2-(aq) â†' 2 Mn^2+(aq) + 10 CO2(g) + 8 H2O(l).

SOLVED: A solution of sodium oxalate (Na2C2O4) in acidic solution is titrated with a solution of potassium permanganate (KMnO4) according to the following balanced chemical equation: 2KMnO4(aq) + 8H2SO4(aq) + 5Na2C2O4(aq) â†'

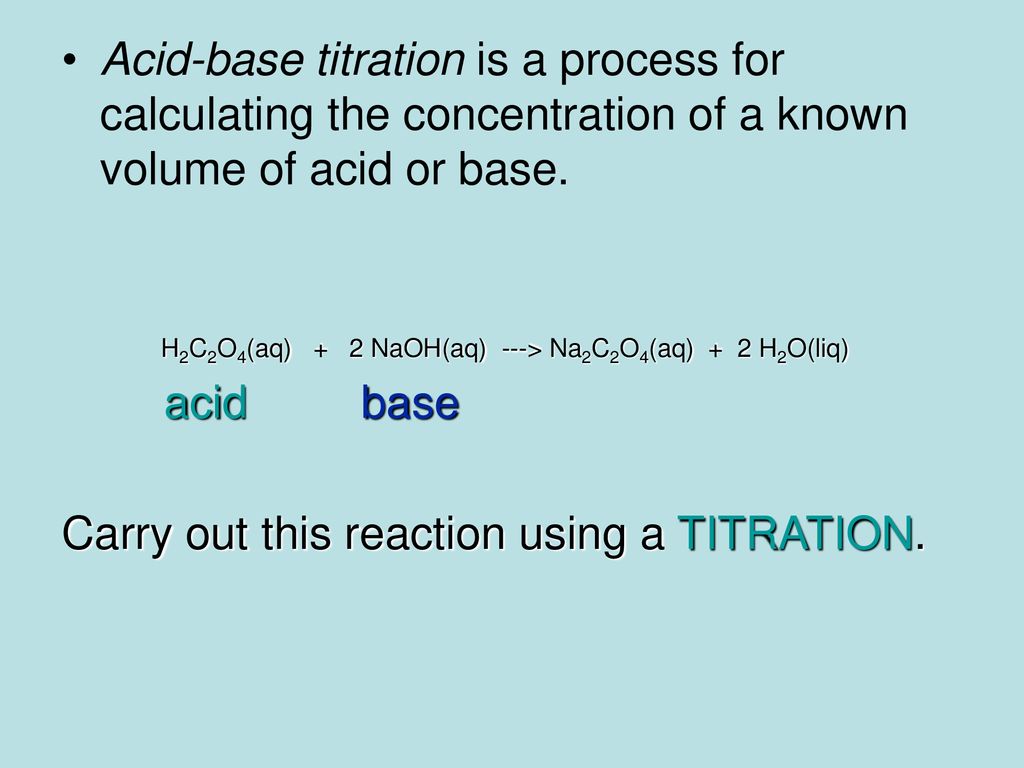
How to Write the Net Ionic Equation for H2C2O4 + NaOH = Na2C2O4 + H2O | How to Write the Net Ionic Equation for H2C2O4 + NaOH = Na2C2O4 + H2O Did
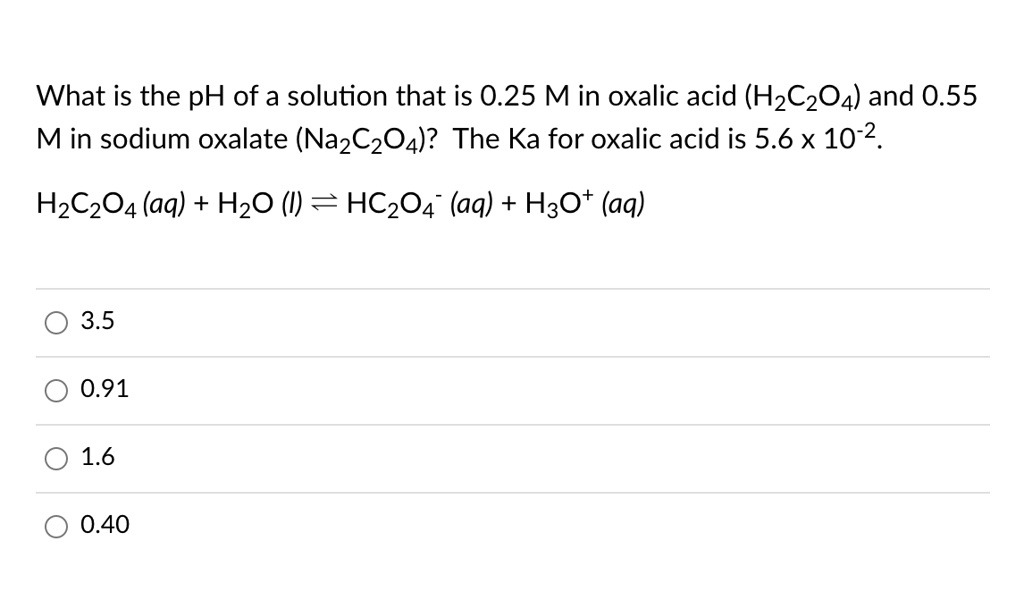
SOLVED: What is the pH of a solution that is 0.25 M in oxalic acid (H2C2O4) and 0.55 M in sodium oxalate (Na2C2O4)? The Ka for oxalic acid is 5.6 x 10^-2.
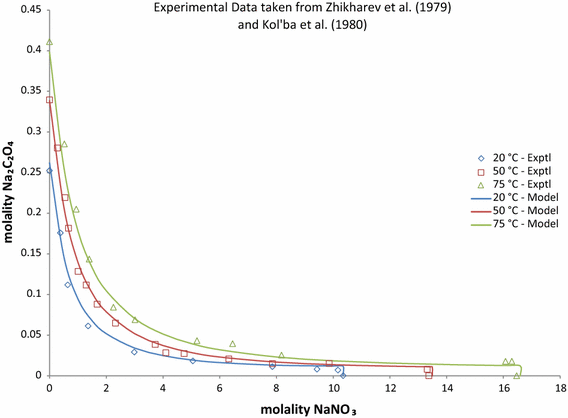
Pitzer Model Anion–Anion and Ternary Interaction Parameters for the Na2C2O4 –NaOH–H2O and Na2C2O4–NaNO3–H2O Systems | Journal of Solution Chemistry

SOLVED: A solution of sodium oxalate (Na2C2O4) in acidic solution is titrated with a solution of potassium permanganate (KMnO4) according to the following balanced chemical equation: 2 KMnO4 (aq) + 8 H2SO4 (

SOLVED: A 0.1278 g sample of primary standard Na2C2O4 is diluted to 100.00 mL with water and made slightly acidic before titration with a potassium permanganate solution of unknown concentration. The titration