How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of MnO2 ? Zn + 2MnO2 + H2O - brainly.com
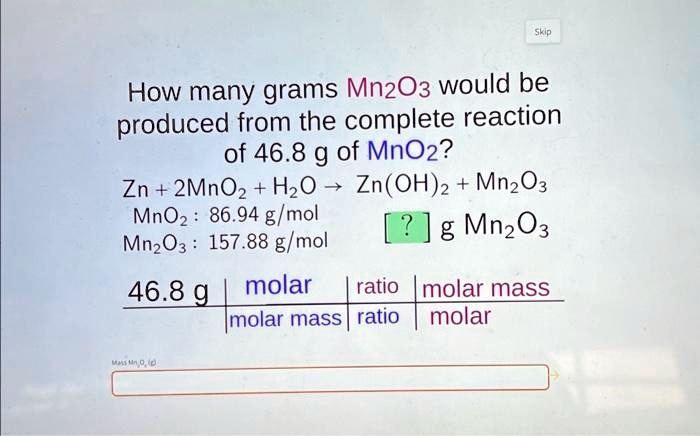
SOLVED: How many grams of Mn2O3 would be produced from the complete reaction of 46.8 g of MnO2? Zn + 2MnO2 + H2O -> Zn(OH)2 + Mn2O3 MnO2: 86.94 g/mol Mn2O3: 157.88

Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts, and single crystalline ultra-long Na4Mn9O18 nanowires – topic of research paper in Nano-technology. Download scholarly article PDF and read for ...

Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports
How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of MnC 2 ? Zn+ [algebra]

Irregularly Shaped Mn2O3 Nanostructures with High Surface Area for Water Oxidation | ACS Applied Nano Materials

Kinetics of Mn2O3–Mn3O4 and Mn3O4–MnO Redox Reactions Performed under Concentrated Thermal Radiative Flux | Energy & Fuels
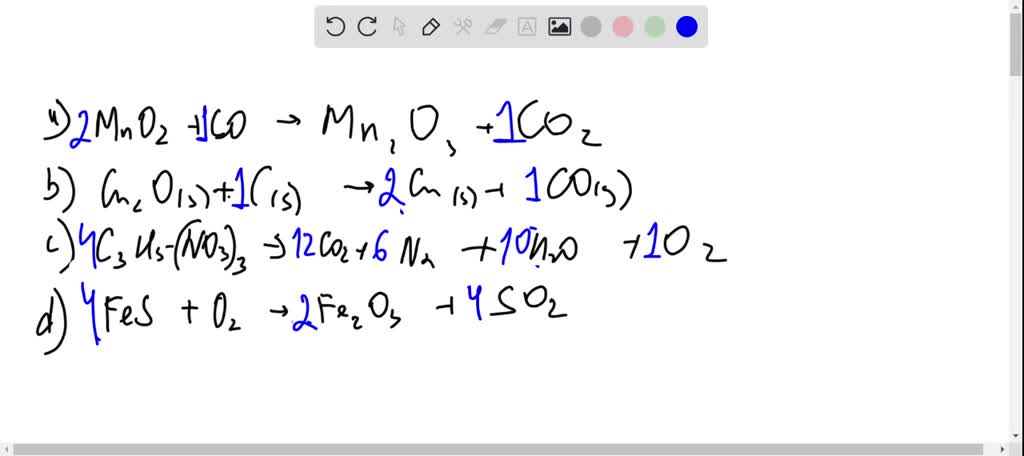
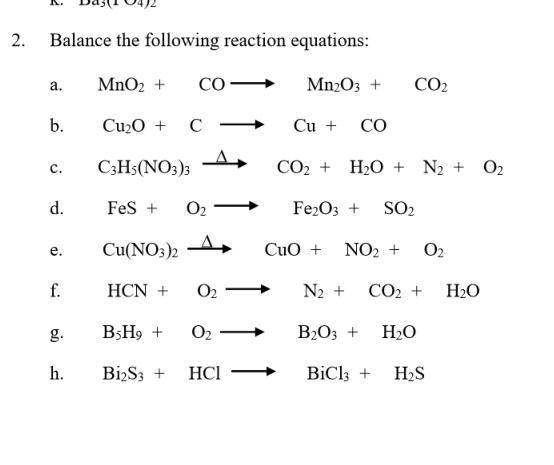
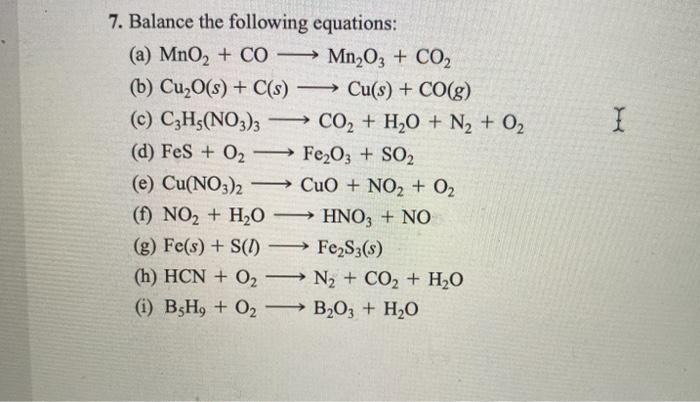
SOLVED: Balance the following equations: (a) MnO2 + CO â†' Mn2O3 + CO2 (b) Cu2O(s) + C(s) â†' Cu(s) + CO(g) (c) C3H5(NO3)3 â†' CO2 + H2O + N2 + O2 (d)

Evaluation of MnOx, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction of Water | The Journal of Physical Chemistry C
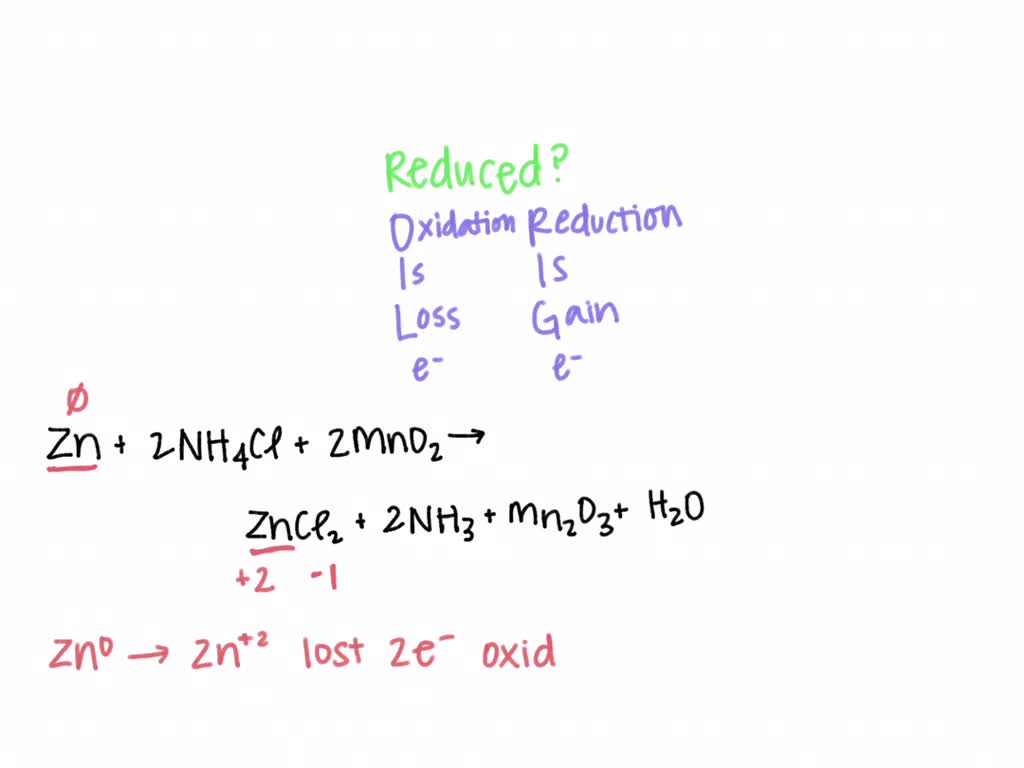
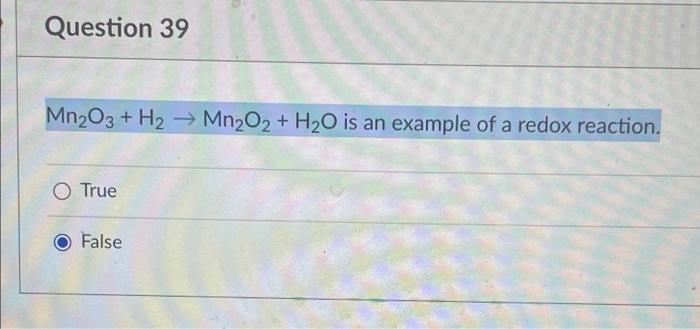
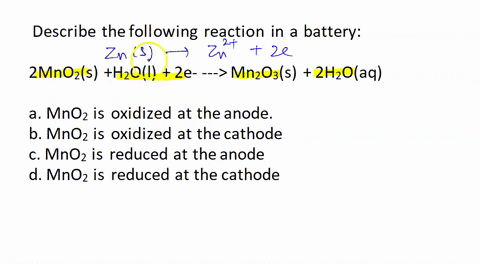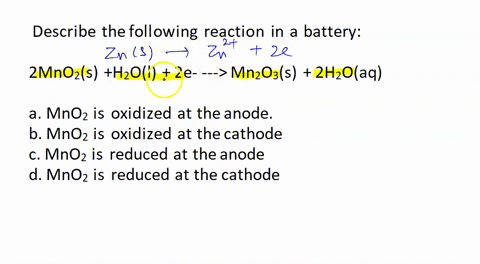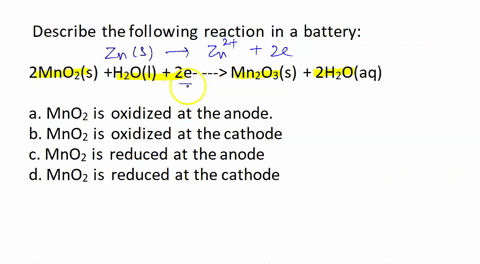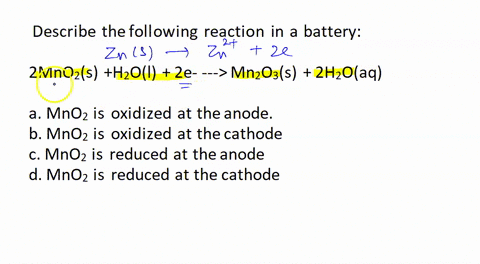
SOLVED: In an acid dry cell battery for which the reaction is Zn + 2 NH4Cl + 2 MnO2 ? ZnCl2 + 2 NH3 + Mn2O3 + H2O, what atom is reduced?

Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports
Proposed transformation pathways of BPA in the Mn2O3/PMS system. First,... | Download Scientific Diagram

Tuning Single-Atom Dopants on Manganese Oxide for Selective Electrocatalytic Cyclooctene Epoxidation | Journal of the American Chemical Society
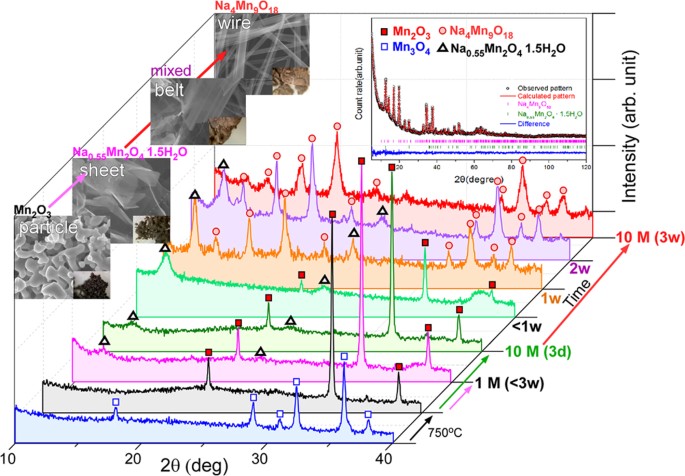
Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports
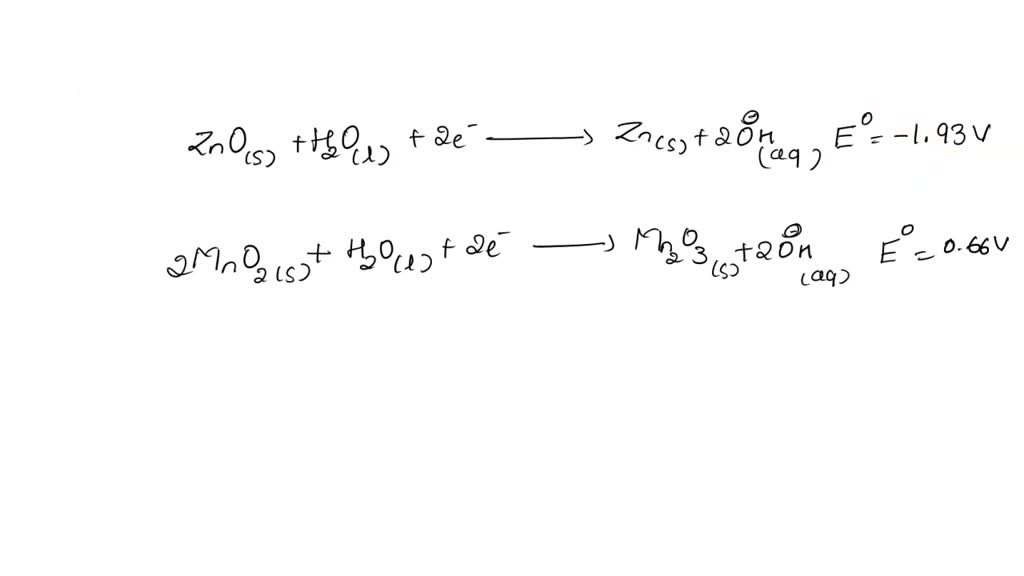
SOLVED: The standard reduction potentials of the half-reactions in single-use alkaline batteries are: ZnO(s) + H2O(l) + 2e- –> Zn(s) + 2OH-(aq) Eo = -1.93V 2MnO2(s) + H2O(l) + 2e- –> Mn2O3(s) +

XANES spectra of Mn K-edge; (a) Mn2O3, (b) Mn3O4, (c) MnO2, (d) MnO,... | Download Scientific Diagram
![Solved [3] Manganese dry cell Cathode 2MnO2 + 2H+ + 2e → | Chegg.com Solved [3] Manganese dry cell Cathode 2MnO2 + 2H+ + 2e → | Chegg.com](https://media.cheggcdn.com/study/b83/b839ebec-1828-4793-8e66-88ddd87ee165/image)