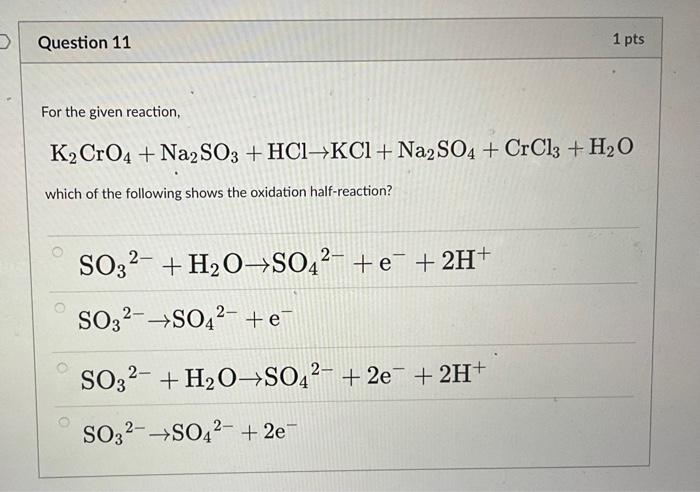
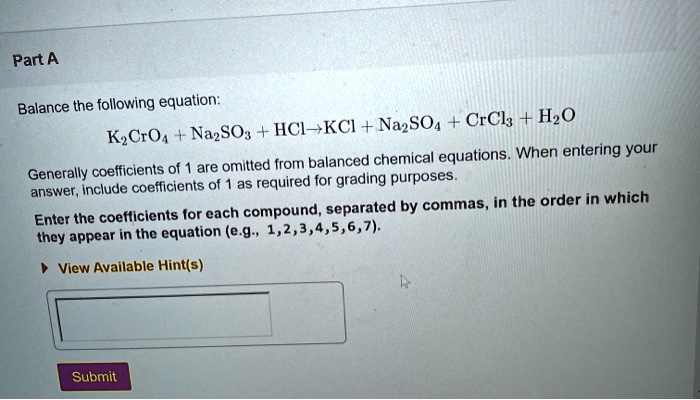
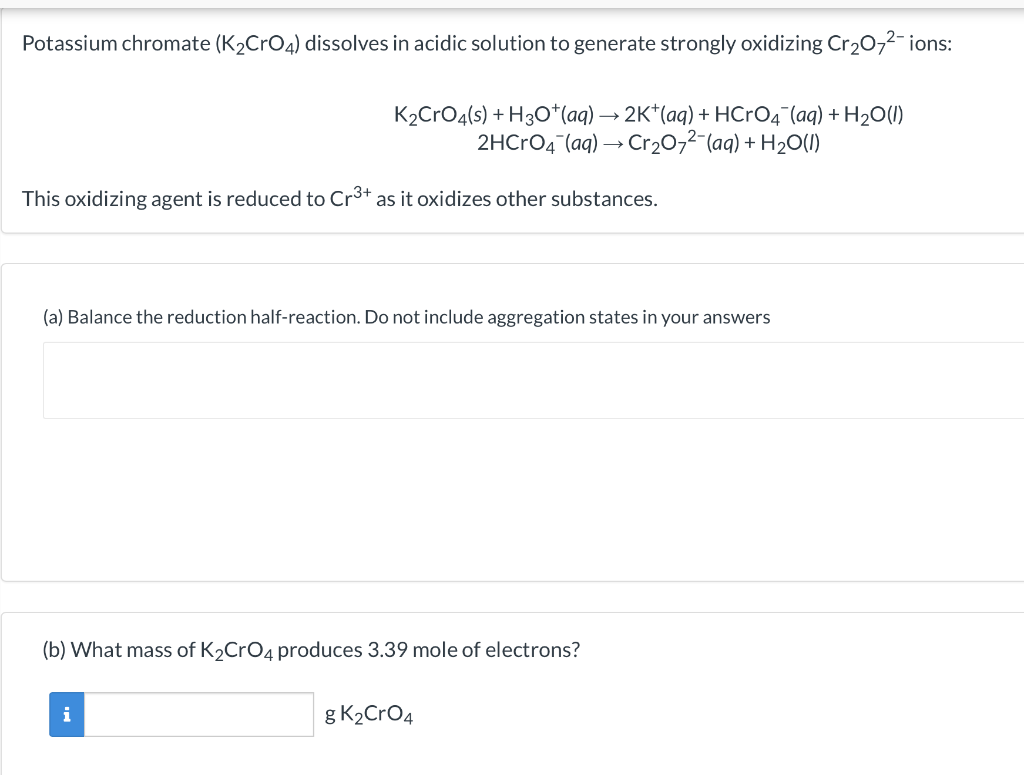
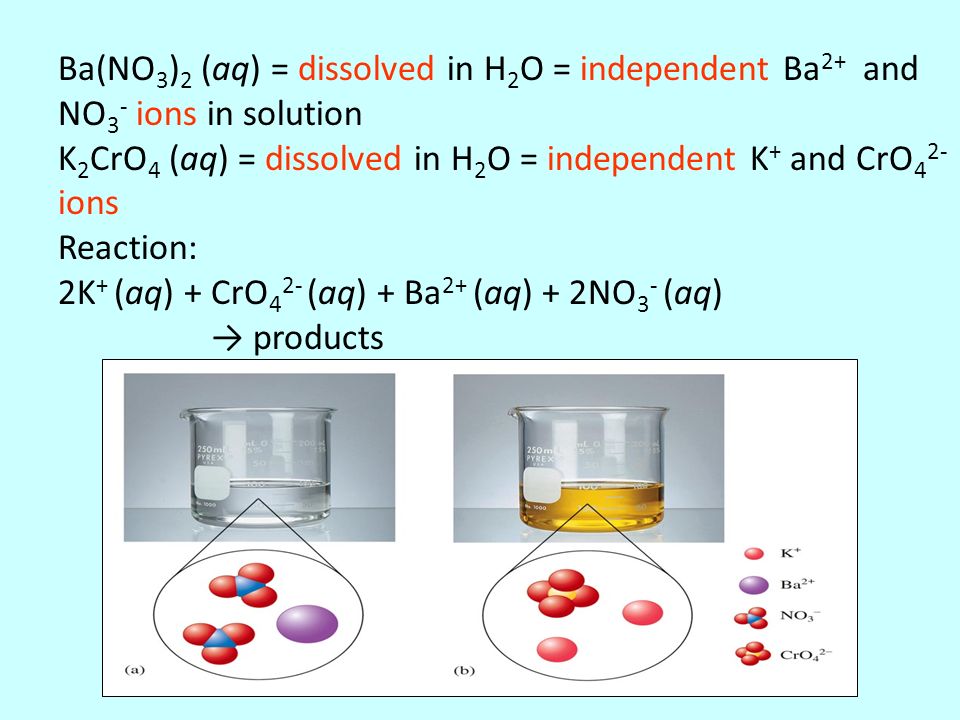
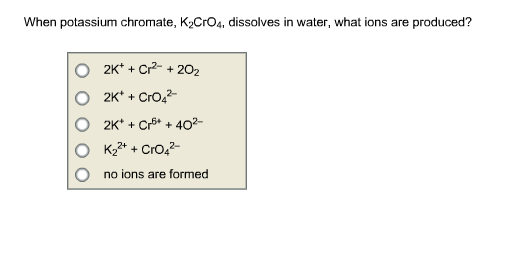
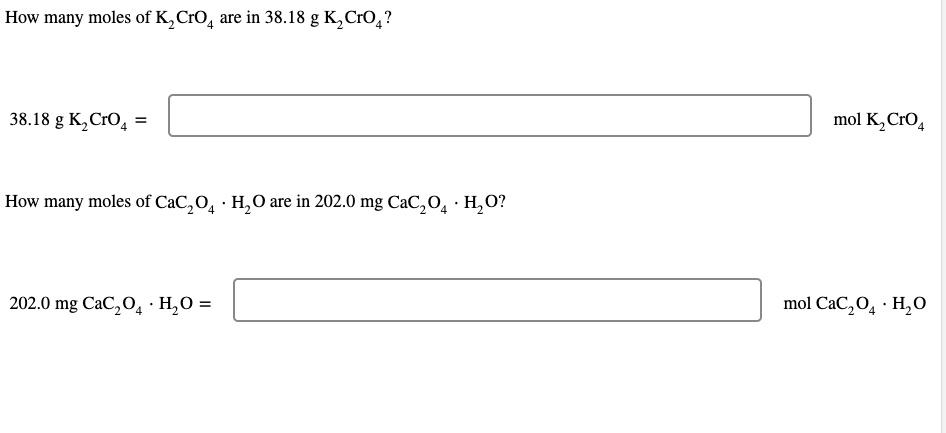
SOLVED: A standard aqueous solution of potassium chromate (K2CrO4, MW = 194.2 g/mol) is prepared by dissolving 106.3 g of K2CrO4 in 400.0 g of water (H2O, MW = 18.02 g/mol). The

K2CrO4 + K2SO3 + H20 -> Cr(OH)3 + K2SO4+ KOH Uzupełnij współczynniki Bilansem jonowo-elektronowym. - Brainly.pl
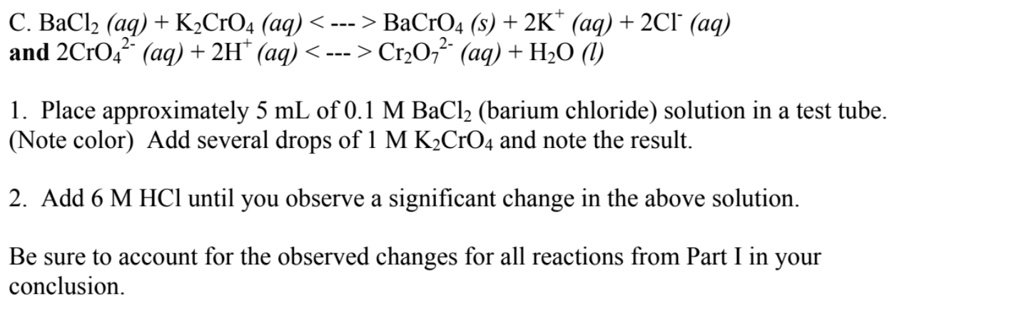
SOLVED: C.BaCl2 (aq) + K2CrO4 (aq) -> BaCrO4 + 2KCl (aq) 2CrO4^2- (aq) + 2H+ (aq) -> Cr2O7^2- (aq) + H2O Place approximately 5 mL of 0.1 M BaCl2 (barium chloride) solution
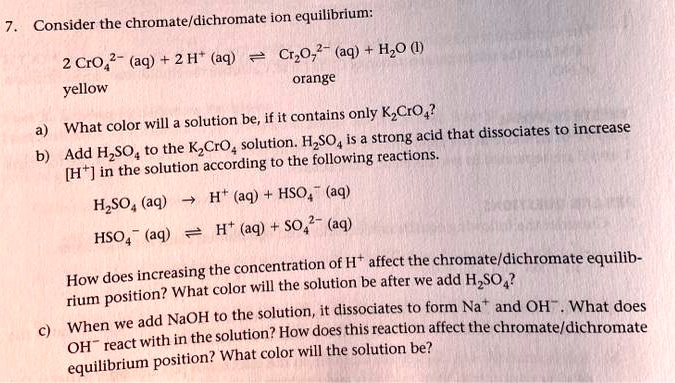
SOLVED: Consider the chromate/dichromate ion equilibrium: CrO4^2- (aq) + H2O (l) ⇌ 2 CrO4^2- (aq) + 2 H+ (aq). The solution will be orange-yellow if it contains only K2CrO4. What color will

Solubility of the System KOH–K2CrO4–Al2O3–H2O at 150 °C in a High Alkali Concentrated Region | Journal of Chemical & Engineering Data
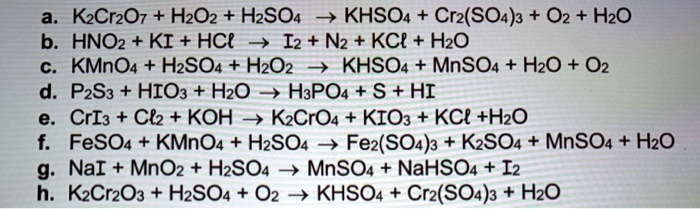
SOLVED: K2Cr2O7 + H2O2 + H2SO4 -> KHSO4 + Cr2(SO4)3 + O2 + H2O HNO2 + KI + HCl -> I2 + N2 + KCl + H2O KMnO4 + H2SO4 -> H2O2 +