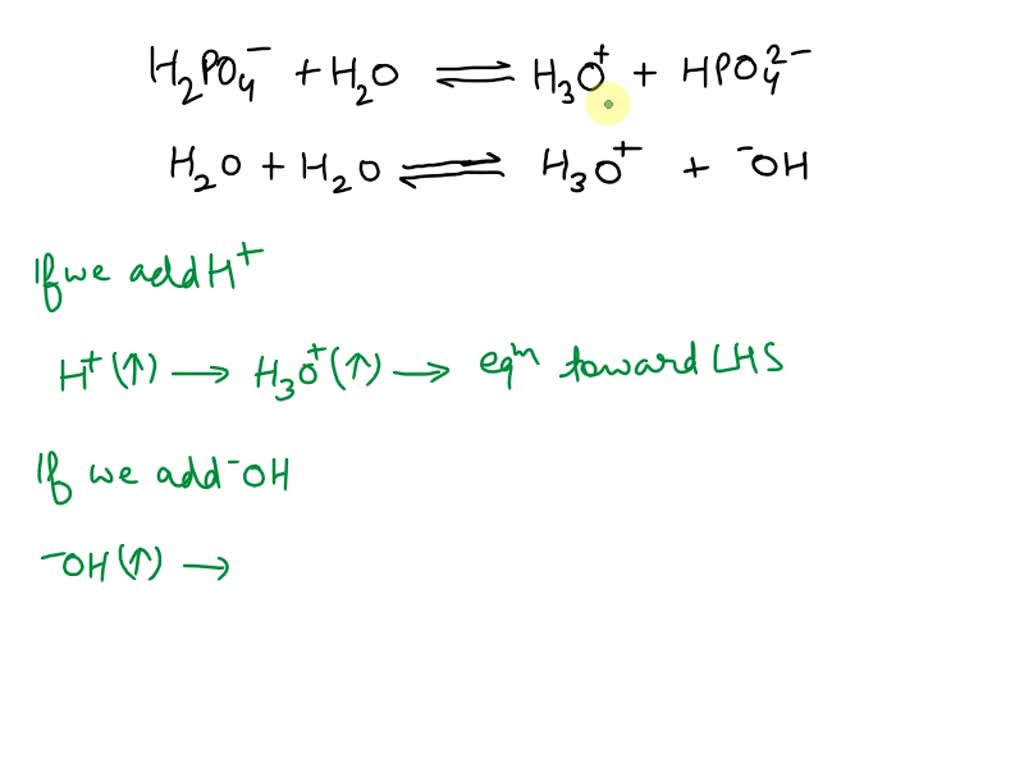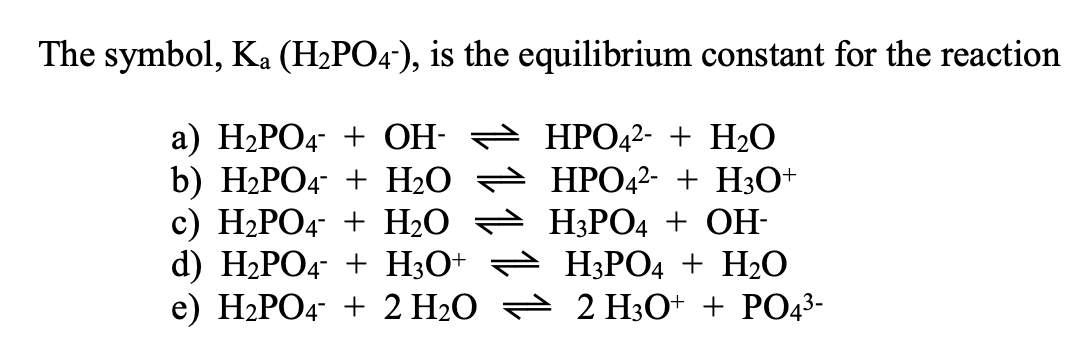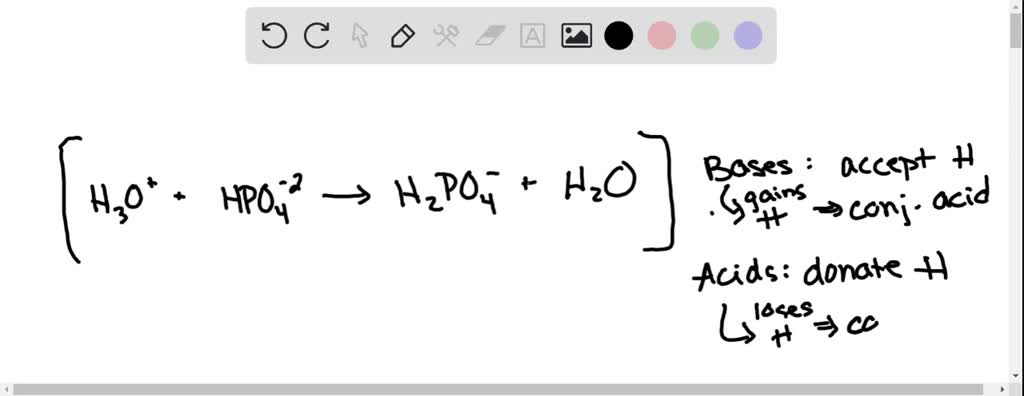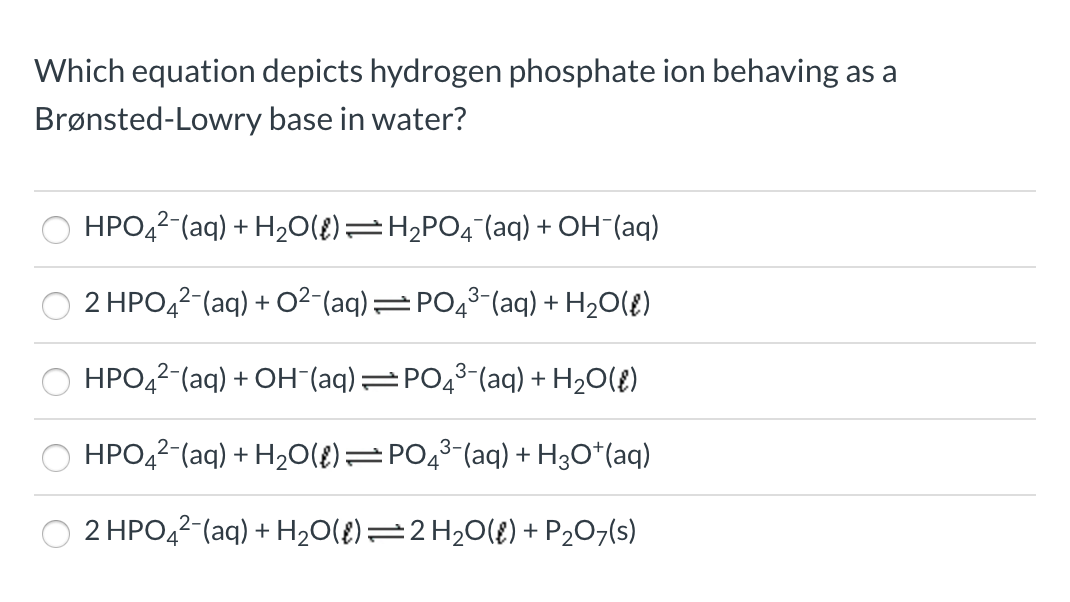PDF) Thermal decomposition kinetics and reversible hydration study of the Li2Zn(HPO4)2�H2O | Chanaiporn Danvirutai - Academia.edu

A new process for Na2Ca(HPO4)2 synthesis and its application as a heterogeneous catalyst in Knoevenagel condensation | Chehab | Mediterranean Journal of Chemistry

Figure 6 from VIIIVIV(HPO4)4·enH·H2O: a mixed-valence vanadium phosphate with an open framework | Semantic Scholar
![Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries](https://pubs.acs.org/cms/10.1021/acs.inorgchem.2c03308/asset/images/medium/ic2c03308_0004.gif)
Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries

Figure 5. XRD of Zr0.8Ti0.2(HPO4)2.H2O : α- Zirconium Titanium Phosphates - Fibrous Cerium Phosphate Composite Membranes and Their 1,10- Phenanthroline Cu(II) Pillared Materials : Science and Education Publishing

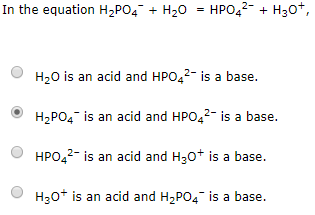
SOLVED: Choose balanced equation for the transfer of a proton between dihydrogen phosphate ion and the hydroxide ion: H2PO4-(aq) + OH-(aq) â†' HPO4 2-(aq) + H2O(l)
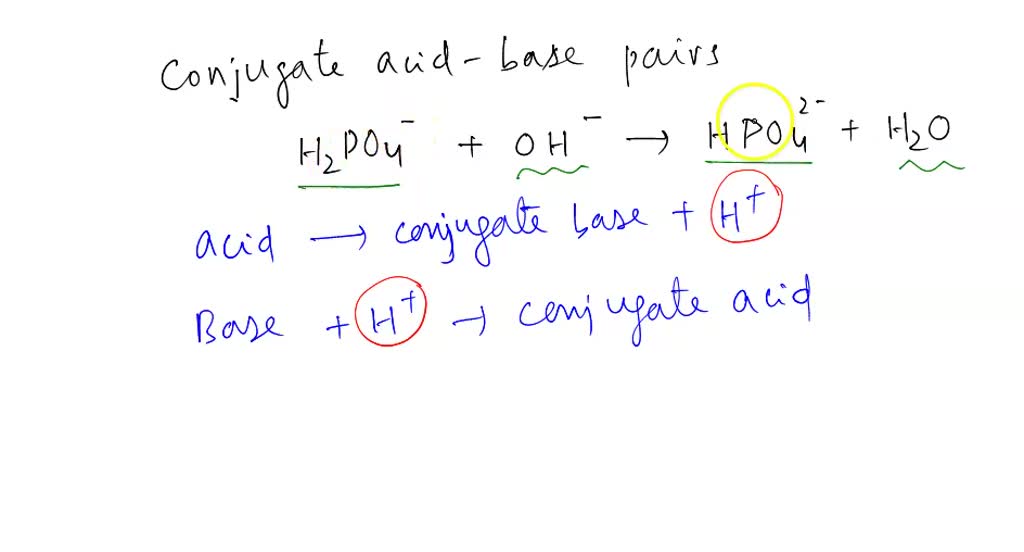
SOLVED: B. Identify the conjugate acid-base pairs in the following reactions: H2PO4- + OH- → HPO4-2 + H2O HBr + H2O → H3O+ + Br- CO3-2 + H2O → HCO3- + OH-

Catalytic conversions of isocyanate to urea and glucose to levulinate esters over mesoporous α-Ti(HPO4)2·H2O in green media - X-MOL

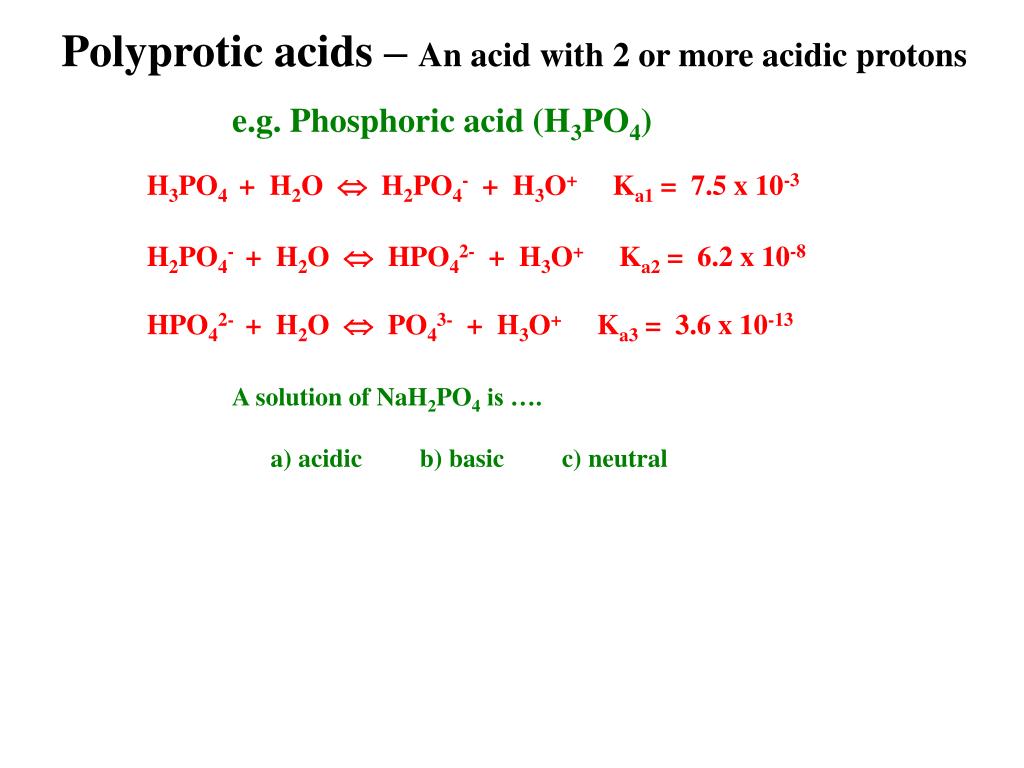
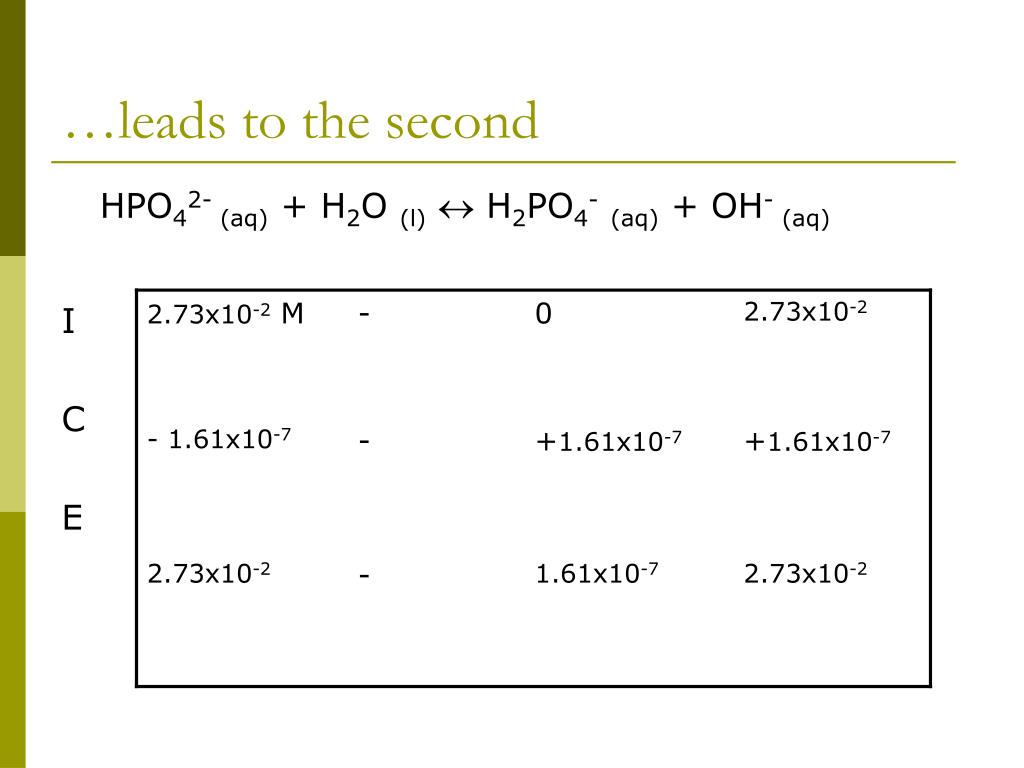
SOLVED: 7. Consider the following equilibrium equations: HPO4 + H2O ↔ H2PO4- + OH- H2PO4- + H2O ↔ HPO4 2- + H3O+ HPO4 2- + H2O ↔ H2PO4- + OH- (1) (2) (