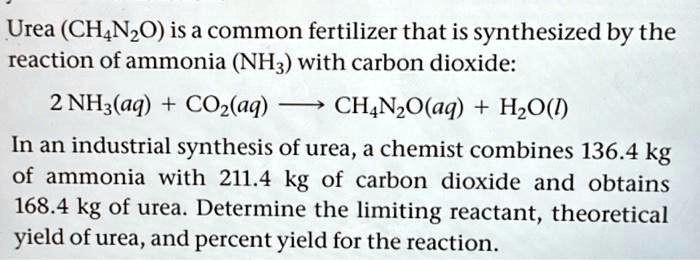
SOLVED: Texts: Urea (CH4N2O) is a common fertilizer that is synthesized by the reaction of ammonia (NH3) with carbon dioxide (CO2): 2 NH3(aq) + CO2(aq) -> CH4N2O(aq) + H2O(l) In an industrial

What is the solution's freezing point: 15 g of CH4N2O (Molar mass = 60.055 g/mol) in 200. g of H2O? (Kf = - brainly.com

NaCl·CH4N2O·H2O: An Organic–Inorganic Hybrid Ultraviolet Nonlinear Optical Crystal with Optimized Comprehensive Properties | Inorganic Chemistry

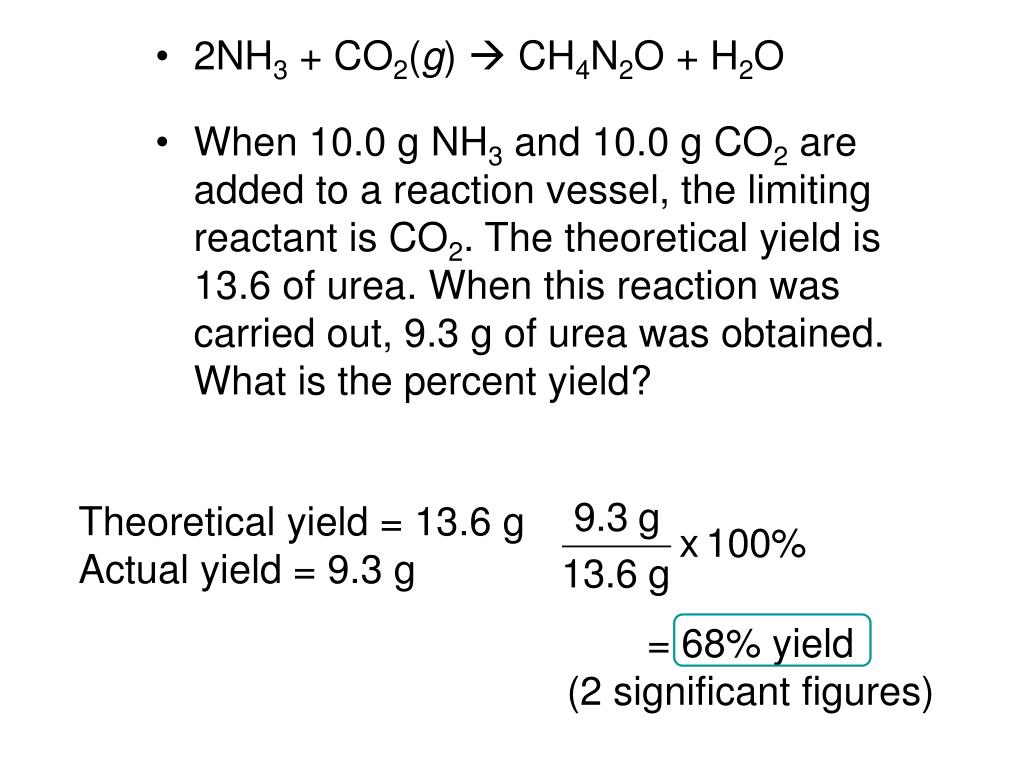
SOLVED: Given the following balanced reaction: 2 NH3 + CO2 â†' CH4N2O + H2O. A reaction produces 87.5 g of CH4N2O upon the reaction of 68.2 g of NH3 with 105 g

SOLVED: Urea (CH4N2O) is a common fertilizer that can be synthesized by the reaction of ammonia with carbon dioxide according to the following equation: NH3 + CO2 â†' CH4N2O + H2O (equation

SOLVED: Urea (CH4N2O) is a common fertilizer that is synthesized by the reaction of ammonia (NH3) with carbon dioxide: 2 NH3(aq) + CO2(aq)-CH4N2O(aq) + H2O(l) In an industrial synthesis of urea, a
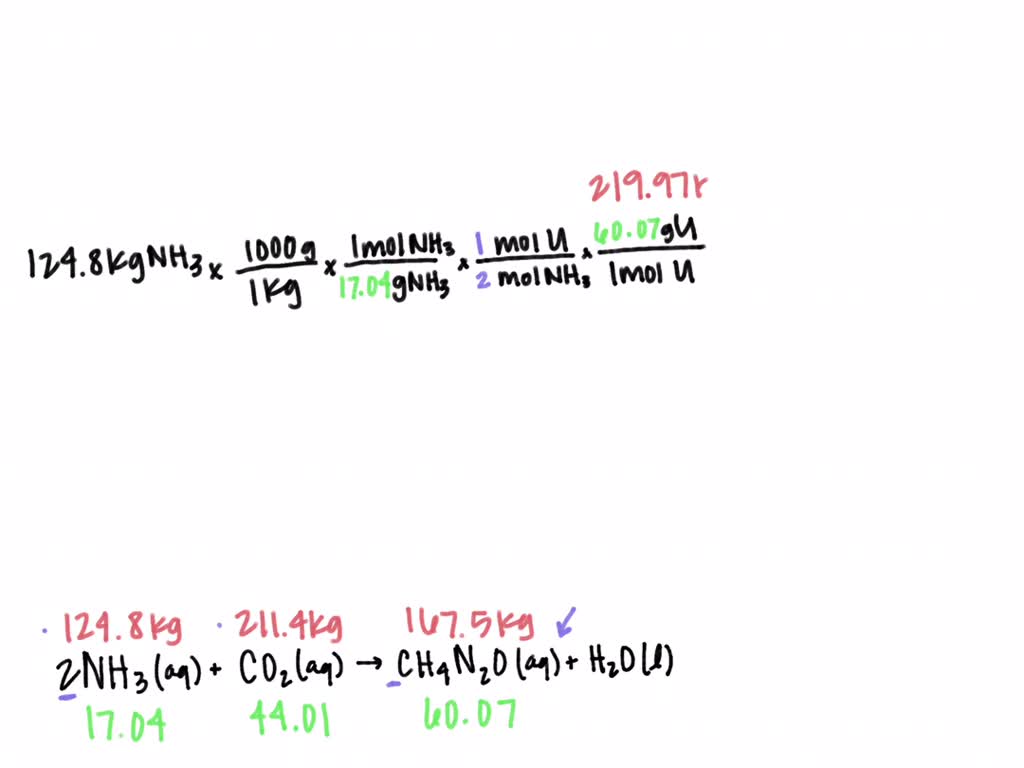
SOLVED: Urea (CH4N2O) is a common fertilizer that can be synthesized by the reaction of ammonia (NH3) with carbon dioxide as follows: 2NH3(aq)+CO2(aq)→ CH4N2O(aq)+H2O(l) In an industrial synthesis of urea, a chemist combines
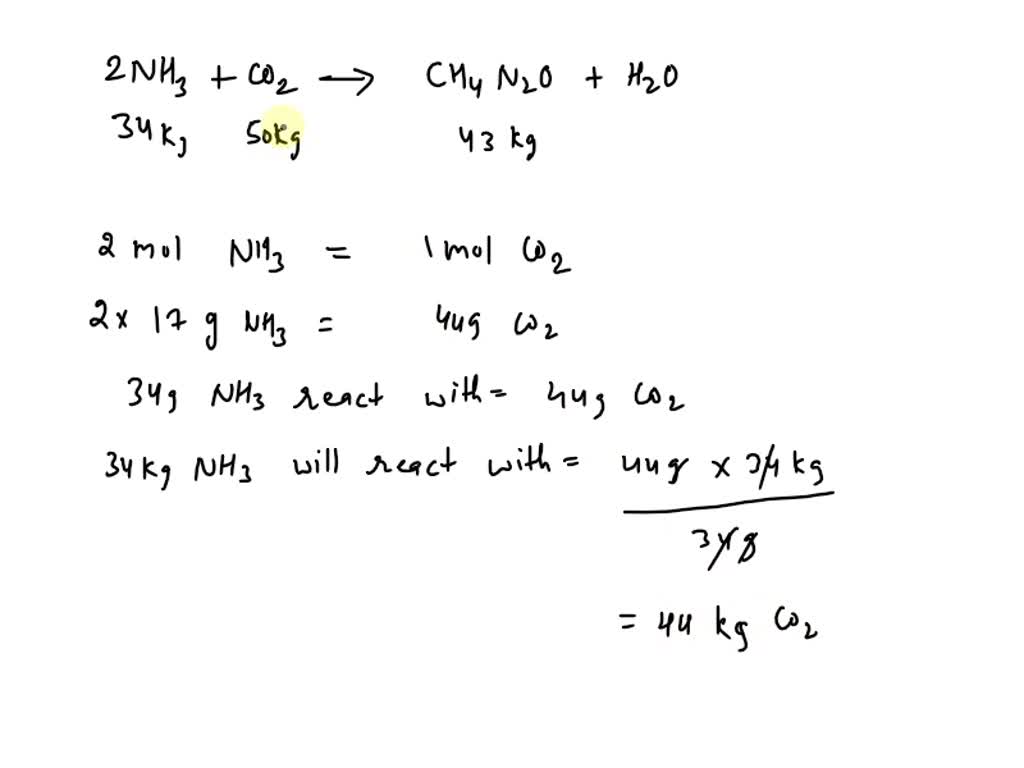
SOLVED: Urea (CH4N2O) is a common fertilizer that can be synthesized by the reaction of ammonia with carbon dioxide according to the following equation: NH3 + CO2 â†' CH4N2O + H2O (equation
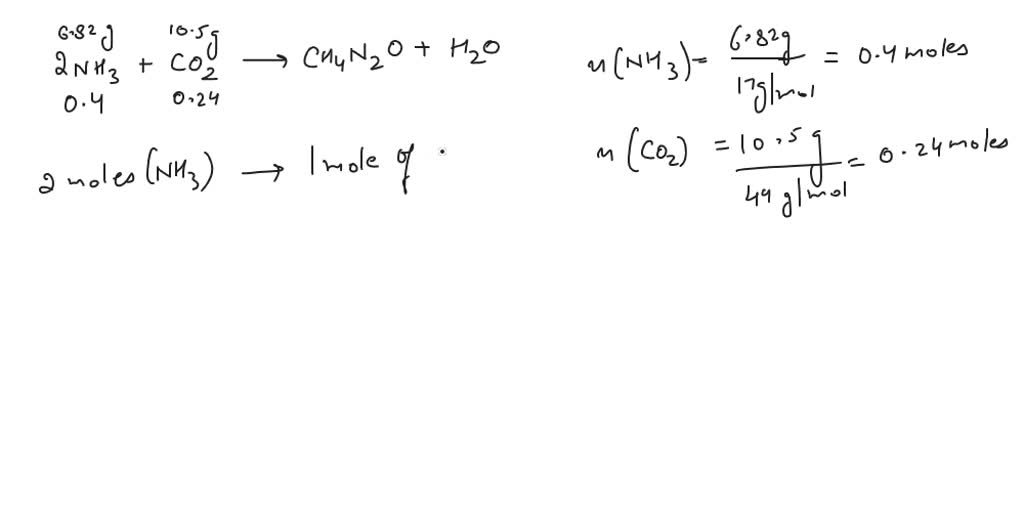
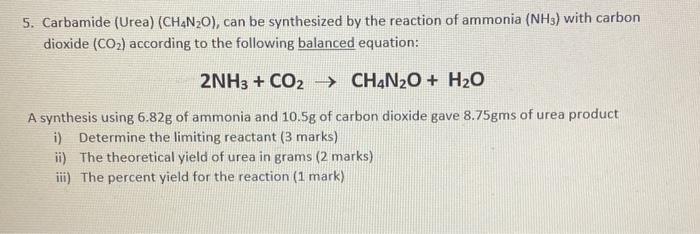
SOLVED: Carbamide (Urea) (CH4N2O), can be synthesized by the reaction of ammonia (NH3) with carbon dioxide (CO2) according to the following balanced equation: 2NH3 + CO2 -> CH4N2O + H2O A synthesis