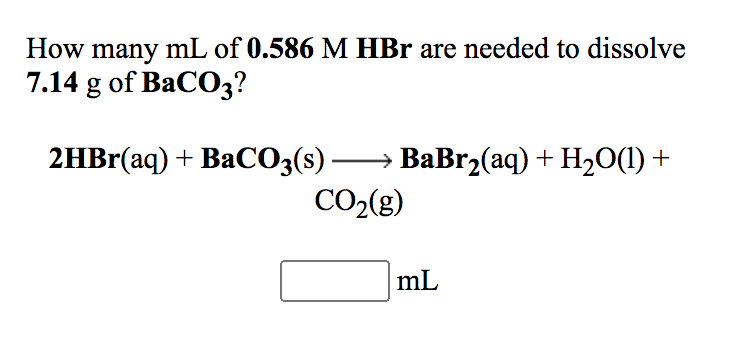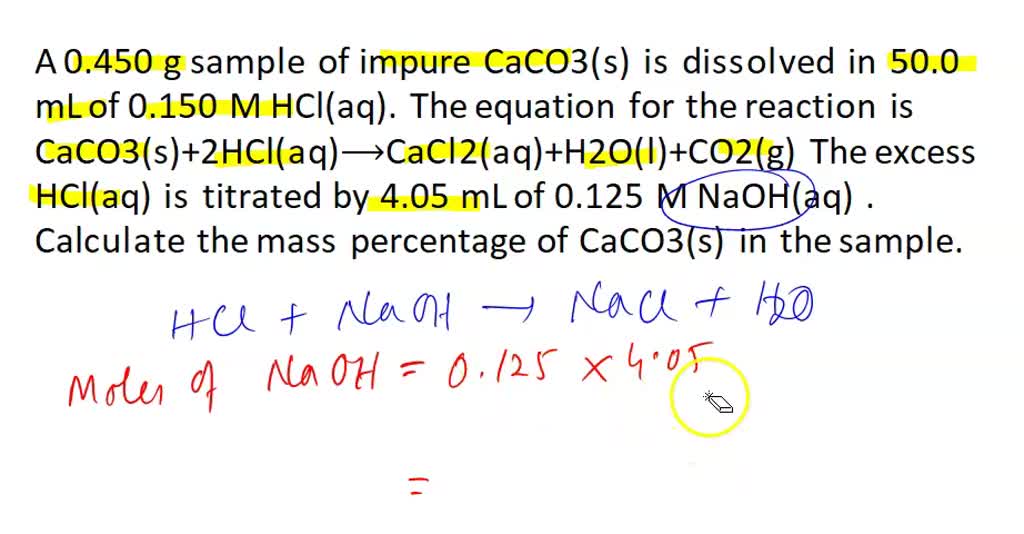
SOLVED: (b) The Ksp of barium carbonate, BaCO3, is 2.58×10^(-9). Calculate the molar solubility, S, of this compound. S= (c) A 0.450 g sample of impure CaCO3(s) is dissolved in 50.0 mL

Date twe of bor WYSICAL SUENCE Works Balance the following equations: 1. AL + N2 - AIN 2. Fe + 02 - Fe3O4 Caco - CaO + CO2 NH.NO, N2O + H2O

PPT - What is the difference between a chemical reaction and physical change? PowerPoint Presentation - ID:5813241

The experimental enthalpies of solution of Y, BaCO3 and CoCl2·4.24H2O... | Download Scientific Diagram

24.Write equilibrium constant expression the following reac (i) BaCO3 (8) ---------- BaO(s) + CO2 (g).

Cho sơ đồ các phản ứng theo đúng tỉ lệ mol:(a) X(t0) $ \to $ Y + CO2(b) Y + H2O $ \to $ Z(c) T + Z $ \to $ R + X + H2O(d?

Synthetic pathway of 1. a) NH2OH·HCl, BaCO3, Pd/C, N2H4·H2O, reflux in... | Download Scientific Diagram