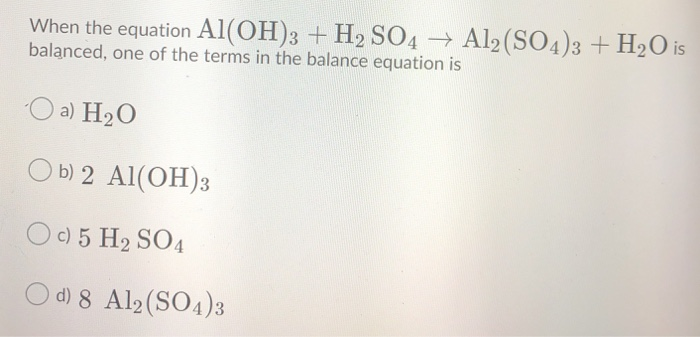Non-Ferric Aluminium Sulphate (Formula: Al2 (SO4) 3 X H2O (X 14-18) - China Nonferric, Iron Free | Made-in-China.com

Phase Diagrams of (NH4)2SO4–Al2(SO4)3–H2O Ternary System: Effect of Sulfuric Acid and Its Application in Recovery of Aluminum from Coal Fly Ash | Journal of Chemical & Engineering Data
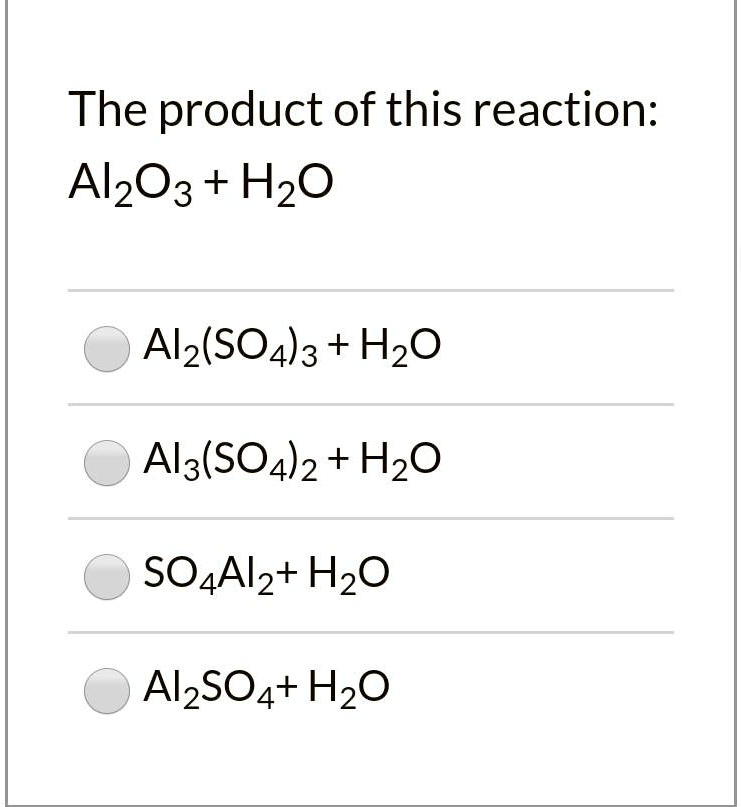
SOLVED: The product of this reaction: Al2O3 + H2O Al2(SO4)3 + H2O Al3(SO4)2 + H2O SO4Al2 + H2O Al2SO4 + H2O

Alumminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3+H2SO4-->Al2(SO4)+6H2O. Which reagent is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How ma | Homework.Study.com

Aluminium sulphate solution 0.2 % Al2(SO4)3 * 18 H2O for blue number determination Contents:, 48,91 €

OneClass: Mixing 39.0g of Al(OH)3 with an excess of H2SO4 we produce Al2(SO4)3. Using the following e...
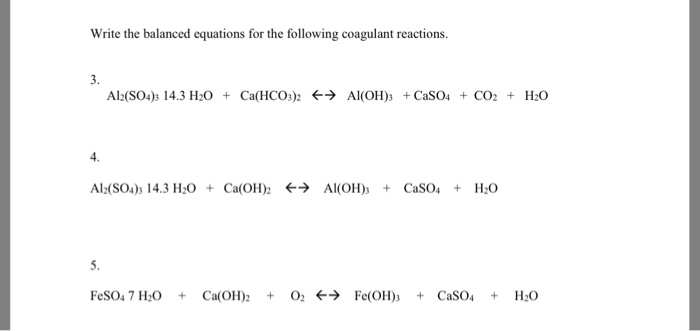
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3 + NO H2O H2S + K2Cr2O7 + H2SO4 =KHSO4 + Cr2(SO4)3 + S + H2O Balance the equations using ion electron method.

Solved) - Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l) Express your answer... (1 Answer) | Transtutors

Aluminium sulphate solution 30 % Al2(SO4)3 * 18 H2O for Blue Number determination Contents: 2, 529,55 €
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3 + NO H2O H2S + K2Cr2O7 + H2SO4 =KHSO4 + Cr2(SO4)3 + S + H2O Balance the equations using ion electron method.
:no_upscale()/3000.jpg)
19516.3000 - Aluminium sulfate solution, 40 % Al2(SO4)3 * 18 H2O/l, technical grade, 1 L | Analytics-Shop

![Punjabi] Balance the chemical equation : Al(OH)3 + H2SO4 → Al2(SO4)3 Punjabi] Balance the chemical equation : Al(OH)3 + H2SO4 → Al2(SO4)3](https://static.doubtnut.com/ss/web-overlay-thumb/10301342.webp)