Jual Aluminium (III) Sulfat, 18-hidrat | Al2(SO4)3.18H2O 500 gram - Kota Bandung - Rofa Laboratorium Centre | Tokopedia

Al2(SO4)3.18H2O after calcium for 2 h at 500-1300ºC with the weight of... | Download Scientific Diagram

Consider the reaction of Al2O3 withH2SO4 to form Al2(SO4)3 and H2O. If 3.84 g AL2O3 is reacted with excess - brainly.com

Aluminium sulphate solution 0.2 % Al2(SO4)3 * 18 H2O for blue number determination Contents:, 48,91 €

SRL Aluminium Sulphate Octadecahydrate extrapure, 98%, 500Gm, CAS NO 7784-31-8, Molecular Formula : Al2(SO4)3.18H2O, Storage : Room Temperature Shelf Life : 60 Months for laboratory and industrial use : Amazon.in: Industrial & Scientific

Phase Diagrams of (NH4)2SO4–Al2(SO4)3–H2O Ternary System: Effect of Sulfuric Acid and Its Application in Recovery of Aluminum from Coal Fly Ash | Journal of Chemical & Engineering Data
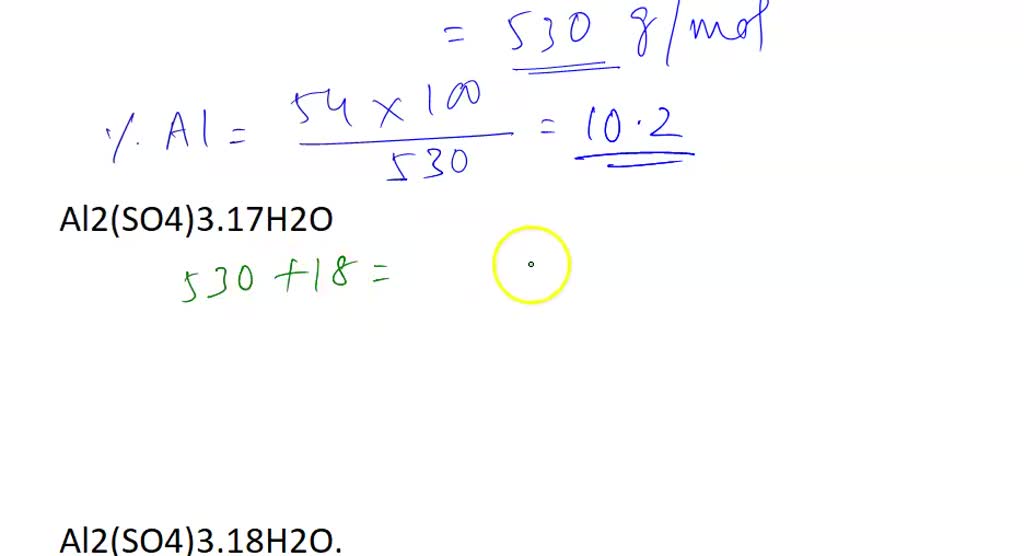
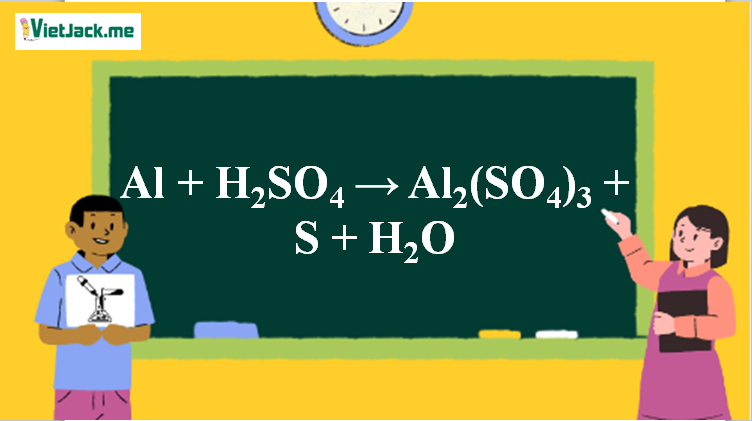
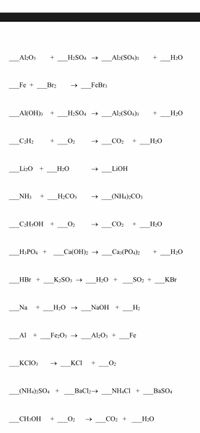
SOLVED: Aluminum sulfate occurs as a hydrate, commonly with 16-18 moles of H2O. Calculate the molar masses and Al% for each Al2(SO4)3*16H2O, Al2(SO4)3*17H2O, and Al2(SO4)3*18H2O.
ALUMINIUM SULPHATE practical iron free Extra Pure | Lab chemical distributors, Lab chemicals exporter, Laboratory chemical suppliers, Lab chemical supplier, Laboratory Chemicals, Laboratory chemicals manufacturer, Lab chemical manufacturer, Alpha ...